Shown to improve accuracy and speed of measurement compared to traditional methods
Lexington, Mass., February 26, 2024 – FUJIFILM Healthcare Americas Corporation, a leading provider of endoscopic imaging and endosurgical solutions, has been granted FDA 510(k) clearance for SCALE EYE, a new endoscopic imaging technology and part of its expanding ELUXEO Endoscopic Imaging System. Consisting of a laser-equipped colonoscope (model EC-760S-A/L) and endoscopy support software (EW10-VM01), the SCALE EYE system displays a linear or circular virtual measurement – or scale – over the area of interest on the endoscopy monitor. Accessible at the push of a button, SCALE EYE aids endoscopists in estimating the size of colorectal lesions in vivo without relying on visual estimations, consumable tools, or the need for additional surgical instruments.
Traditionally, endoscopists estimate the size of neoplasms by comparing them against the size of the forceps being used to examine them, which creates risk for subjectivity. Compared to the biopsy forceps method, SCALE EYE enables more accurate,1 objective measurement of colon polyp size, a critical factor in making decisions for clinical management of neoplasms – specifically colonic polyps, as size can be an indicator of malignancy. Polyp size measurements are important for risk stratification, choice of polypectomy technique, and follow-up interval decisions.
“During colonoscopy, it is important to correctly measure the size of the polyps because it can directly impact the patient’s care pathway,” says Tai Fujita, vice president, Endoscopy Division, FUJIFILM Healthcare Americas Corporation. “Early clinical results of SCALE EYE are impressive, and we’re proud that Fujifilm’s new, innovative in vivo scaling capability is demonstrating success in improving both the speed and accuracy of polyp measurement.”
Several studies have demonstrated the advantages of virtual scale endoscopes (VSE) compared to current methods in use.1-5 Early results of Fujifilm’s SCALE EYE show its superiority compared to traditional open biopsy forceps:6
“When it comes to polyps, accurate size assessment is essential to our decision-making process. Polyp size is a factor in determining a patient’s recommended follow-up interval and may impact the decision on how best to achieve a safe and complete resection. We’re encouraged by early data and are excited to be the first U.S. site to evaluate SCALE EYE,” said Dr. Seth Gross, clinical chief of the Department of Gastroenterology and Hepatology, NYU Langone Health.”
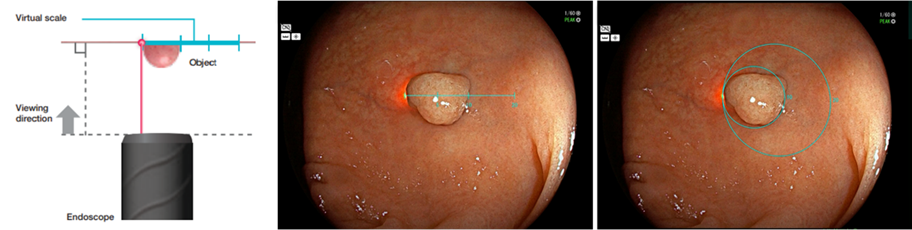
The Fujifilm endoscope is equipped with a laser, and the position of the laser changes according to the distance between the tip of endoscope and the object. Fujifilm’s ELUXEO® system software detects the position of the laser in the endoscopic image, displays the virtual scale on the image and provides a numerical value for the scale on the right side. Endoscopists can compare the size of an object with the virtual scale by positioning the laser spot on the left edge of the object.
Fujifilm will commercially launch SCALE EYE in 2024 following completion of a limited market evaluation. To learn more about SCALE EYE, contact Fujifilm here: https://us.fujimed.com/SCALEEYE
SCALE EYE and ELUXEO are registered trademarks of FUJIFILM Corporation in various jurisdictions.
About Fujifilm
FUJIFILM Healthcare Americas Corporation is a comprehensive healthcare company that has an extensive range of technology and expertise in the detection, diagnosis and treatment of diseases. Fujifilm’s innovative medical imaging portfolio includes solutions for digital radiography, mammography, CT, MRI, ultrasound, gastroenterology, pulmonology, endosurgery, and minimally invasive surgery. The award-winning Synapse® Enterprise Imaging portfolio provides healthcare professionals with the cross-departmental imaging and data access needed to deliver a complete patient record. Fujifilm’s AI initiative, REiLI®, combines Fujifilm’s rich image-processing heritage with cutting-edge innovations to inspire clinical confidence and combat burnout. The In-Vitro Diagnostic portfolio provides the gold standard of molecular based immunoassay technology for liver surveillance, cutting edge clinical diagnostic chemicals for leading laboratories and diagnostic chemicals for OEM white labelling products. The company is headquartered in Lexington, Massachusetts. Click here for more information.
FUJIFILM Holdings Corporation, Tokyo, leverages its depth of knowledge and proprietary core technologies to deliver “Value from Innovation” in our products and services in the business segments of healthcare, materials, business innovation, and imaging. Our relentless pursuit of innovation is focused on providing social value and enhancing the lives of people worldwide. Fujifilm is committed to responsible environmental stewardship and good corporate citizenship. For more information about Fujifilm’s Sustainable Value Plan 2030, For the year ended March 31, 2023, the company had global revenues of approximately 2.9 trillion yen (21 billion USD at an exchange rate of 134 yen/dollar). For more information, please visit: www.fujifilmholdings.com.
Contact:
Danielle Brown
914-574-3273
danielle.brown@fujifilm.com