
This session will review the phases of biomarker validation, present the status and data for (protein and liquid biopsy) biomarkers, and discuss ongoing biomarker studies.

Dr. Charlton, Professor of Medicine University of Chicago discussed “Case Based HCC Screening and Surveillance” for CLDF (Chorionic Liver Disease Foundation).

Hepatocellular Carcinoma (HCC) Risk Factors and Surveillance: Enhance Risk Stratification with AFP-L3% and DCP Ju Dong Yang, MD, MS, Staff Physician, Karsh Division of Gastroenterology and Hepatology Cedars Sinai Medical Center 18-Nov-2020
Contents:
– HCC Surveillance Improves Patient Outcome
– How to Use AFP-L3 and DCP as Risk Biomarkers?
– What is AFP-L3?
– What is DCP?
– Biomarker Elevations Before Diagnosis
– Combined Use of AFP-L3 and DCP
– How to Order AFP-L3 and DCP?

Contents:
– Intended Use
– Clinical Utility
– How to Order
– Laboratory Test Codes
– Reimbursement Codes
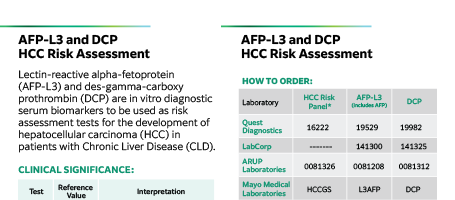
Contents:
– AFP-L3 and DCP HCC Risk Assessment
– Clinical Significance:
– How to Order
– Reimbursement Codes

Contents:
Proposed Algorithm for Surveillance of Hepatocellular Carcinoma
Published in Gastroenterology and Hepatology Volume 10, Issue 2 February 2014

Contents:
A regular surveillance program for patients at risk for development of Hepatocellular Carcinoma (HCC) is recommended by clinical practice guidelines worldwide.

Contents:

Contents:
– Intended Use
– Summary and explanation of the test
– Principle of the method
– Reagents
And more

Contents:
– Intended Use
– Summary and explanation of the test
– Principle of the method
– Reagents
And more
Published content is for information purposes and is intended for healthcare professionals only. The information contained herein is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Where views/opinions are expressed, they are those of the author(s) and not of FUJIFILM.
Please provide the following information to access content:
