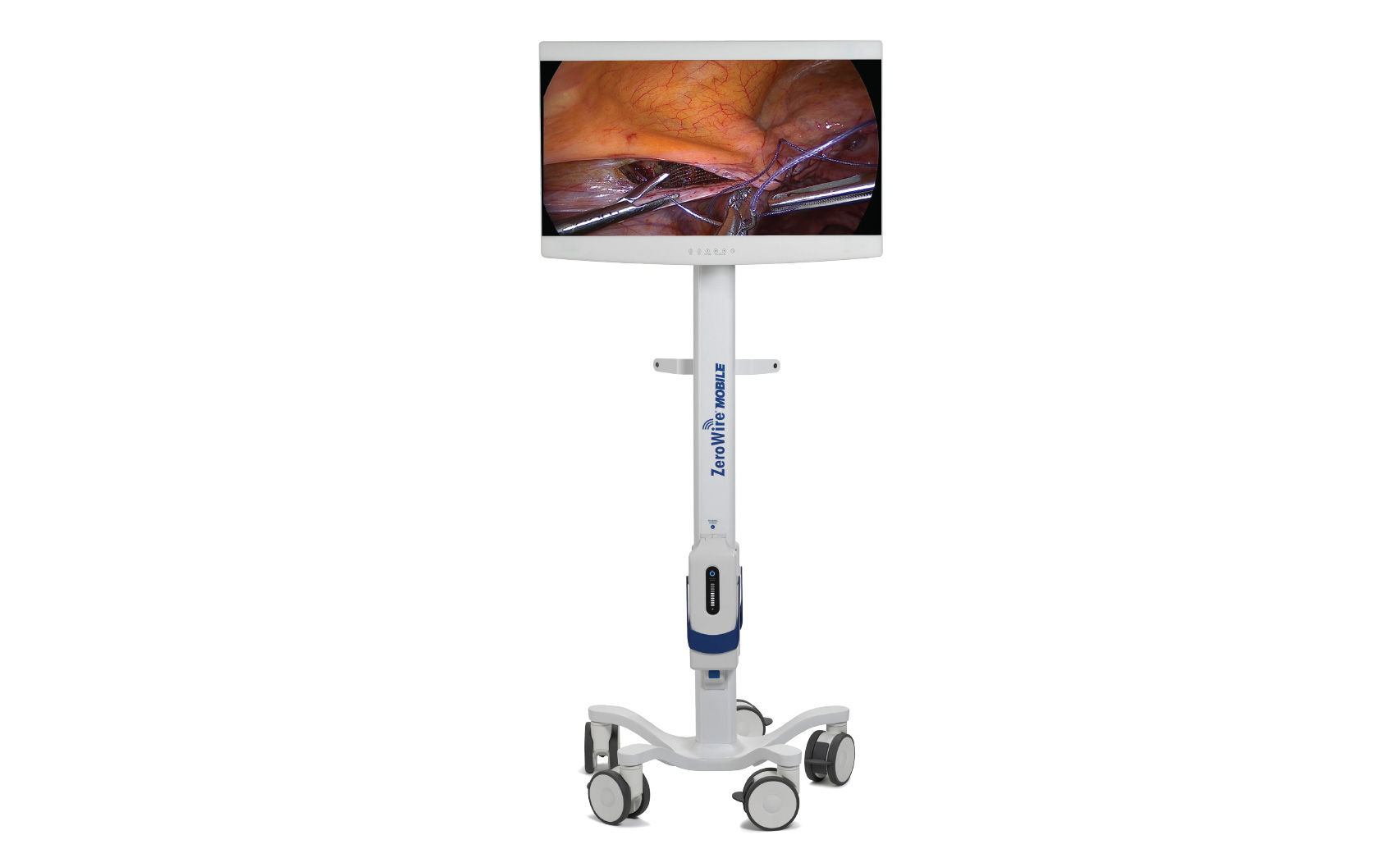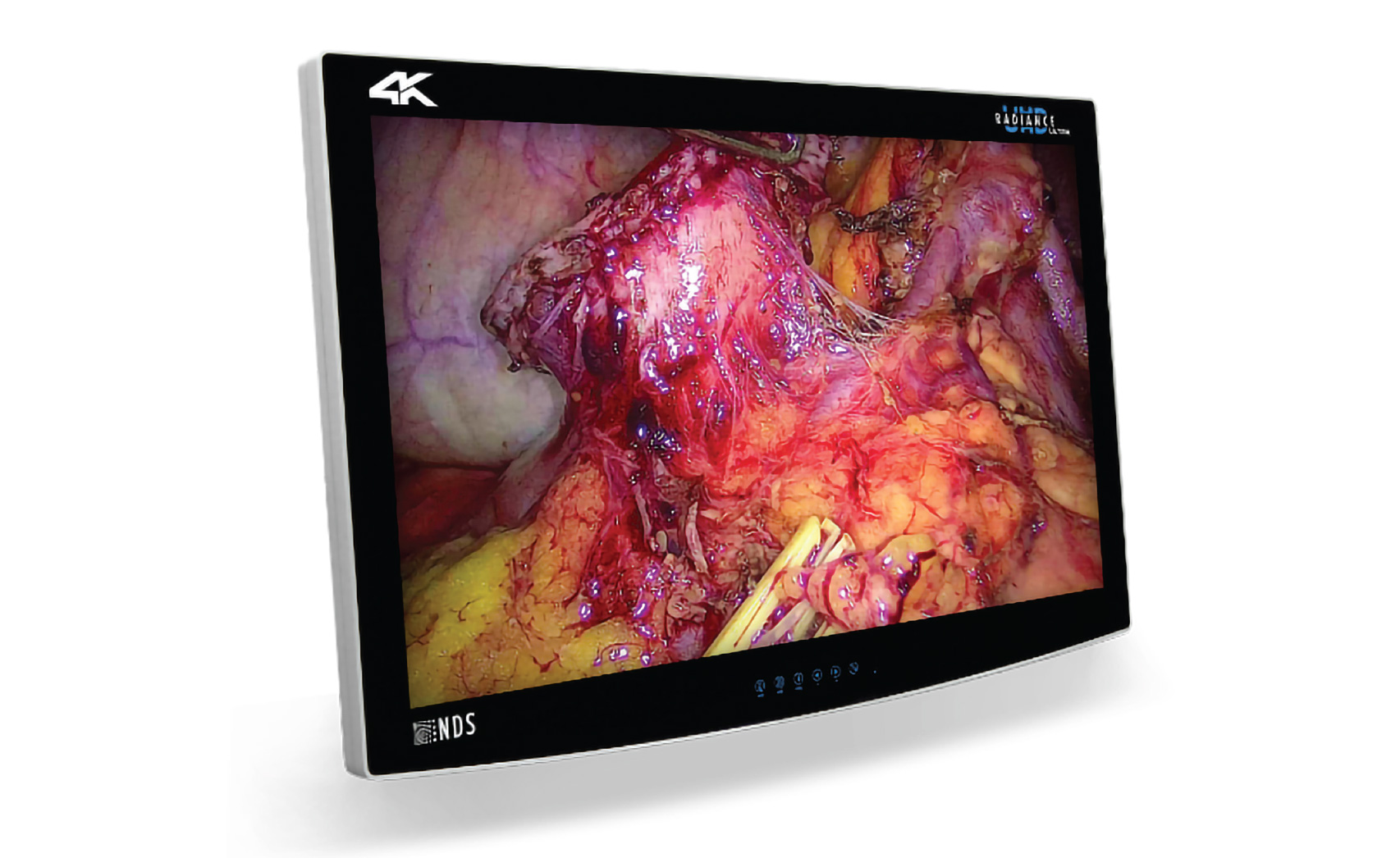
Rely on Radiance Ultra Monitors for Exceptional Visualization
Exclusively engineered by NDS, state-of-the-art TruColor technology found in the Radiance® Ultra TrueColor 32” monitor maximizes 1080p camera images to provide an endoscopic visualization experience that enhances video output with an expanded spectrum of exceptionally vivid colors that more accurately resembles anatomy the human eye would visualize during an open surgical procedure.
TruColor with Medi-Match™ color calibration assures consistent image quality and accurate color reproduction. The result is a 1080p display with the next-generation color performance to rival that of a 4K display.
The Radiance® Ultra 27” endoscopic monitor is engineered with LED backlight technology that produces the brightest typical luminance level to enable deep abdominal illumination, overcoming glare and reflection in high ambient light environments. The Radiance Ultra 27” monitor also comes equipped with Medi-Match™ color calibration to assure consistent image quality and accurate color reproduction. The result is outstanding endoscopic video image performance.
Efficiency and Safety
The Radiance Ultra Series of endoscopic monitors is available with an optional built-in ZeroWire® receiver. When paired with the ZeroWire® Mobile battery-powered stand, the combination becomes the world’s first and only truly cordless and wireless mobile endoscopic solution (patent pending). To eliminate display scratches caused by IV poles or surgical light heads, the Radiance Ultra Series of monitors has scratch-resistant, splash-proof, edge-to-edge glass that includes an industry exclusive 10-year scratch-resistance guarantee.
Product Features
- Optimized for 1080p endoscopy
- Ultra-wide gamut color calibrated
- Advanced imaging capabilities
- Cleanable splash-proof design
- 10-year scratch-resistant-glass guarantee
- ZeroWire® embedded receiver optional
Product Specifications
- Radiance Ultra TruColor 32″ Display w/ Primary Board, ZeroWire Embedded – 90R0115
ESS Radiance Ultra 32″ Monitors
| DESCRIPTION | PRODUCT CODE |
| RU TC 32” with Primary Board | 90R0114 |
| RU TC 32” with Primary and Secondary Digital Board |
90R0112 |
| RU TC 32” with Primary Board and ZeroWire Embedded EndoVue 21” |
90R0115 |
| TECHNICAL DATA | |
| Screen Size | 31.5” diagonal |
| Image Size | 27” x 15”/698 x 393 mm (WxH) |
| Resolution | 1920 x 1080 |
| Aspect Ratio | 16:9 |
| View Angle | 178° |
| Luminance | 650 cd/m2 typical @ 6500°K |
| Contrast Ratio | 1,000:1 (nominal) |
| Numbers of Colors | 16.7 Million |
| Backlight Stabilization | NDS Intelli-Guard™ |
| Color Calibration | NDS Medi-Match™ |
| Color Gamut | 120% of BT.709 |
| Response Time | 10ms |
| Latency | 18ms |
| Video Board Configurations | Primary Board – Digital & Analog – DVI-I and 3G-SDI Input/Output (supports VGA/RGB/SoG) Secondary Board – Digital 2nd DVI-D and 3G-SDI Inputs/Outputs, Analog Component/VG |
| Embedded Wireless Receiver | Optional ZeroWire Embedded Receiver |
| Sealed Glass | Bezel Protected, Edge-to-Edge, Chemically Strengthened |
| Glass Anti-Scratch Warranty | 10-Year Scratch-Resistant Guarantee |
| Dimensions | 30.7” x 20” x 3.4”/780mm x 509mm x 87mm (WxHxD) |
| Weight | 29 lbs/13.2 kg (primary board only) |
| Shipping Weight** | 40 lbs/18.2 kg |
| Shipping Dimensions | 36” x 9” x 29”/ 92 x 23 x 74 cm |
| Green Compliance | REACH, RoHS-2, WEEE |
| Regulatory Compliance | ANSI/AAMI ES60601-1, CAN/CSA C22.2 No. 60601-1, Conflict Minerals, FCC Class B, EN60601-1, EN60601-1-2, CE, MDD 93/42/EEC, Class I Medical Device, Designed to meet IPX6, CCC |
| Regulatory Compliance with ZeroWire Embedded |
ANSI/AAMI ES60601-1, CAN/CSA C22.2 No. 60601-1, FCC Class B, CAN ICES-3(B) / NMB-3(B); EN60601-1, EN60601-1-2, MDD 93/42/EEC, EU Class I Medical Device, (RE-D) Directive 2014/53/EU, this device containsmodule: SII-SK63101, EN 302-567 V2.0.24, CE, US Class II Medical Device, FDA cleared, FCC ID: UK2-SII-SK63101, IC ID: 6705A-SIISK63101, Designed to meet IPX6, 007AA106 Power Supply, 100-240 VAC, 50-60 Hz to 24 VDC, 6.25 A AC power cord, |
| Supplied Accessories | region appropriate User Manual – English with multi-language CD-ROM |
| OPTIONAL ACCESSORIES | |
| 90Z0221a |
Fiber Converter 5 VDC Power Cable |
*Must be installed at factory; not field upgradable. **Shipping weights are approximate.
ESS Radiance Ultra 27″ Monitors
| DESCRIPTION | PRODUCT CODE |
| RU 27” with Primary Board and ZeroWire Embedded Receiver |
90R0109 |
| RU 27” with Primary Board | 90R0104 |
| RU 27” with Primary and Secondary Digital Board |
90R0100 |
| TECHNICAL DATA | |
| Screen Size | 27” diagonal |
| Image Size | 24” x 13”/598 x 336 mm (WxH) |
| Resolution | 1920 x 1080 |
| Aspect Ratio | 16:9 |
| View Angle | 178° |
| Luminance | 900 cd/m2 typical @ 6500°K |
| Contrast Ratio | 1,000:1 (nominal) |
| Numbers of Colors | 1.07 Billion |
| Backlight Stabilization | NDS Intelli-Guard™ |
| Color Calibration | NDS Medi-Match™ |
| Color Gamut | BT.709 or SMPTE-C |
| Response Time | 14ms |
| Latency | 18ms |
| Video Board Configurations | Primary Board – Digital & Analog – DVI-I and 3G-SDI Input/Output (supports VGA/RGB/SoG) Secondary Board – Digital 2nd DVI-D and 3G-SDI Inputs/Outputs, Analog Component/VG |
| Embedded Wireless Receiver | Optional ZeroWire Embedded Receiver* |
| Sealed Glass | Bezel Protected, Edge-to-Edge, Chemically Strengthened |
| Glass Anti-Scratch Warranty | 10-Year Scratch-Resistant Guarantee |
| Dimensions (WxHxD) | 26.7” x 17.5” x 3.3”/678mm x 445mm x 84 mm (WxHxD) |
| Weight | 19.5 lbs/8.9 kg (primary board only) |
| Shipping Weight** | w/ Secondary Digital Board – 32 lbs/14.5 kg w/ Secondary Analog Board – 31 lbs/14 kg |
| Shipping Dimensions | 33” x 9” x 22”/84 x 23 x 56 cm |
| Green Compliance | REACH, RoHS-2, WEEE |
| Regulatory Compliance | ANSI/AAMI ES60601-1, CAN/CSA C22.2 No. 60601-1, Conflict Materials, FCC Class B, EN60601-1, EN60601-1-2, CE, MDD 93/42/EEC, Class I Medical Device, designed to meet IPX6 , CCC |
| Regulatory Compliance with ZeroWire Embedded |
ANSI/AAMI ES60601-1, CAN/CSA C22.2 No. 60601-1, FCC Class B, CAN ICES-3(B) / NMB-3(B); EN60601-1, EN60601-1-2, MDD 93/42/EEC, EU Class I Medical Device, US Class II Medical Device, (RE-D) Directive 2014/53/EU, this device contains module: SII-SK63101, EN 302-567 V2.0.24, CE, FCC ID: UK2-SII-SK63101, IC ID: 6705A-UK2-SII-SK63101, designed to meet IPX6, 007AA106 |
| Supplied Accessories | Power Supply, 100-240 VAC, 50-60 Hz to 24 VDC, 6.25 A AC power cord, region appropriate User Manual – English with multi-language CD-ROM |
| OPTIONAL ACCESSORIES | |
| 990Z0221 |
Fiber Converter 5 VDC Power Cable |
*Must be installed at factory; not field upgradable. **Shipping weights are approximate.