It’s time to rethink what’s possible when it comes to elevating performance and quality in colonoscopy. The true innovation leader in delivering LED multi-light technology, ELUXEO is the endoscopic imaging system trusted today by 1,000s of healthcare facilities across the globe and at 100s of hospitals and ASCs across the US since introduction in 2018. With this expansion, Fujifilm leverages a long history of innovation in Artificial Intelligence to deliver a scalable, future ready platform with an unmatched set of innovative solutions for colonic polyp detection and sizing.
CAD EYE detection system was developed utilizing AI deep learning technology to support real-time detection of colonic mucosal lesions such as polyps and adenomas during colonoscopy procedures.
The system delivers both visual and auditory cues to the physician without disrupting workflow, to help prevent lesion oversight — where lesions are in the view but cannot be detected — as well as on the edge of the observation range.
Watch — and listen to — CAD EYE in action!
Studies indicate CAD EYE can lead to considerable advances in colorectal cancer screening and surveillance1 as it:
Desai M et al. Am J Gastroenterol 2024 Feb 23. doi: 10.14309/ajg.0000000000002664.
Rondonotti et al. Endoscopy 2022 54(12):1171-1179,
 Real-time Detection
Real-time DetectionCAD EYE is aimed to improve real time polyp detection, helping to recognize flat lesions, multiple polyps simultaneously, as well as lesions at the corner of the image. CAD EYE Detection is possible with White Light and LCI (Linked Color Imaging) mode.
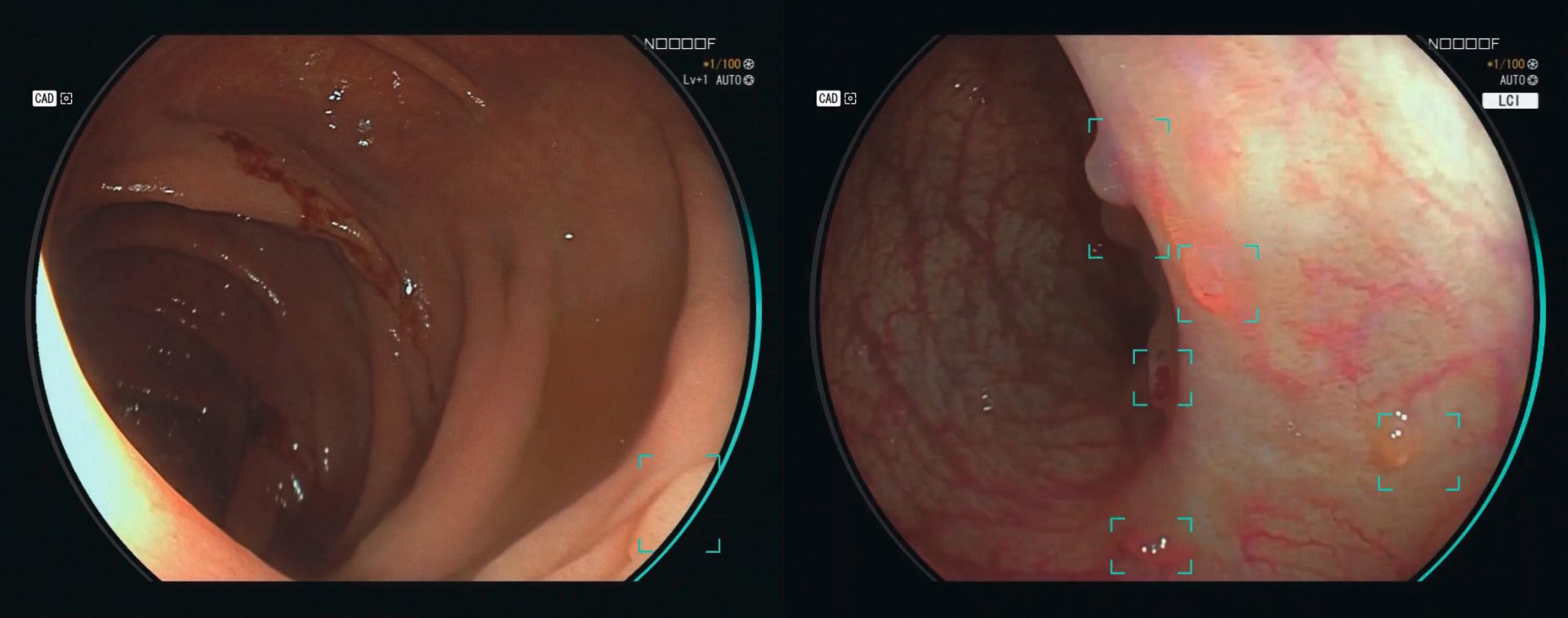
White Light Mode (left) vs LCI Mode (right)
Clinical evidence has shown improved adenoma detection with LCI compared to HD White Light.1
The Effect of Linked Color Imaging for Adenoma Detection. A Meta-analysis of Randomized Controlled Studies. Wang et al. J Gastrointestin Liver Dis. 2022 Mar 19;31(1):67-73. DOI: 10.15403/jgld-4027
Real accuracy for in vivo polyp measurement is here. Polyp size is a major factor in determining a patient’s recommended follow-up interval and may impact the strategy for resection, but with limited measurement tools available, the accepted standard remains visual estimation, making it difficult to ensure accuracy and consistency across all providers. Fujifilm’s SCALE EYE technology aims to address these challenges by delivering objective, real-time measurement support. Using a laser-equipped colonoscope and innovative image processing software, a linear or circular virtual scale can be displayed on the endoscopic image with just the touch of a scope button.
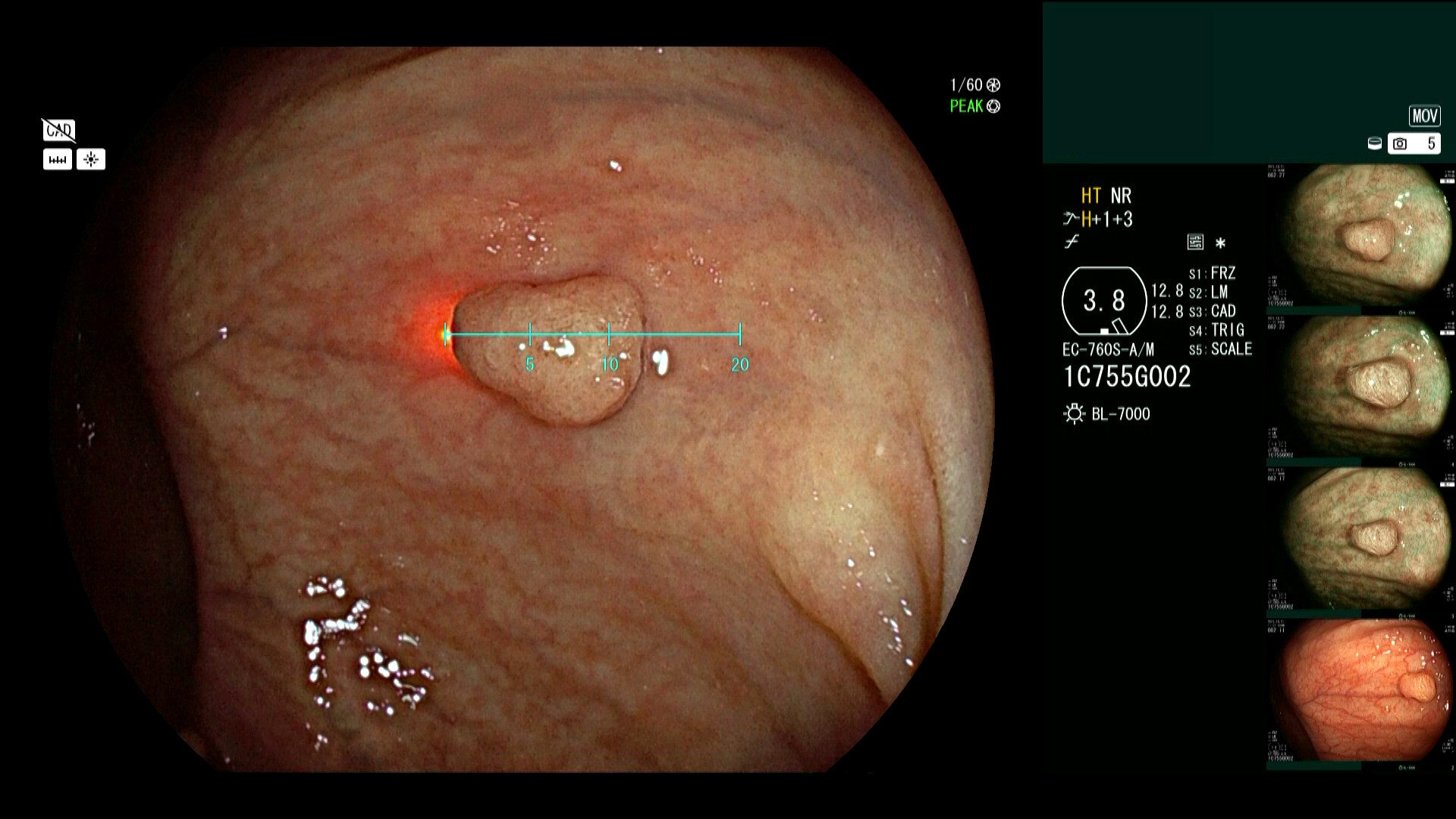
Studies have demonstrated advantages of Fujifilm SCALE EYE virtual scale endoscopes (VSE) compared to current visual estimation methods.
VSE has higher relative accuracy of 85.4% for polyp size measurements compared with visual size estimation at 66.8%.1
The virtual scale function has the potential to standardize measurements among endoscopists.2
von Renteln D, et al. Measuring size of smaller colorectal polyps using a virtual scale function during endoscopies, Gut. 2023 Mar;72(3):417-420.
Shimoda R, et al. Estimating colorectal polyp size with a virtual scale endoscope and visual estimation during colonoscopy: Prospective, preliminary comparison of accuracy Dig Endosc. 2022 Nov;34(7):1471-1477.
Let us help you find the right product or service that will suit your needs.